Clinical trials
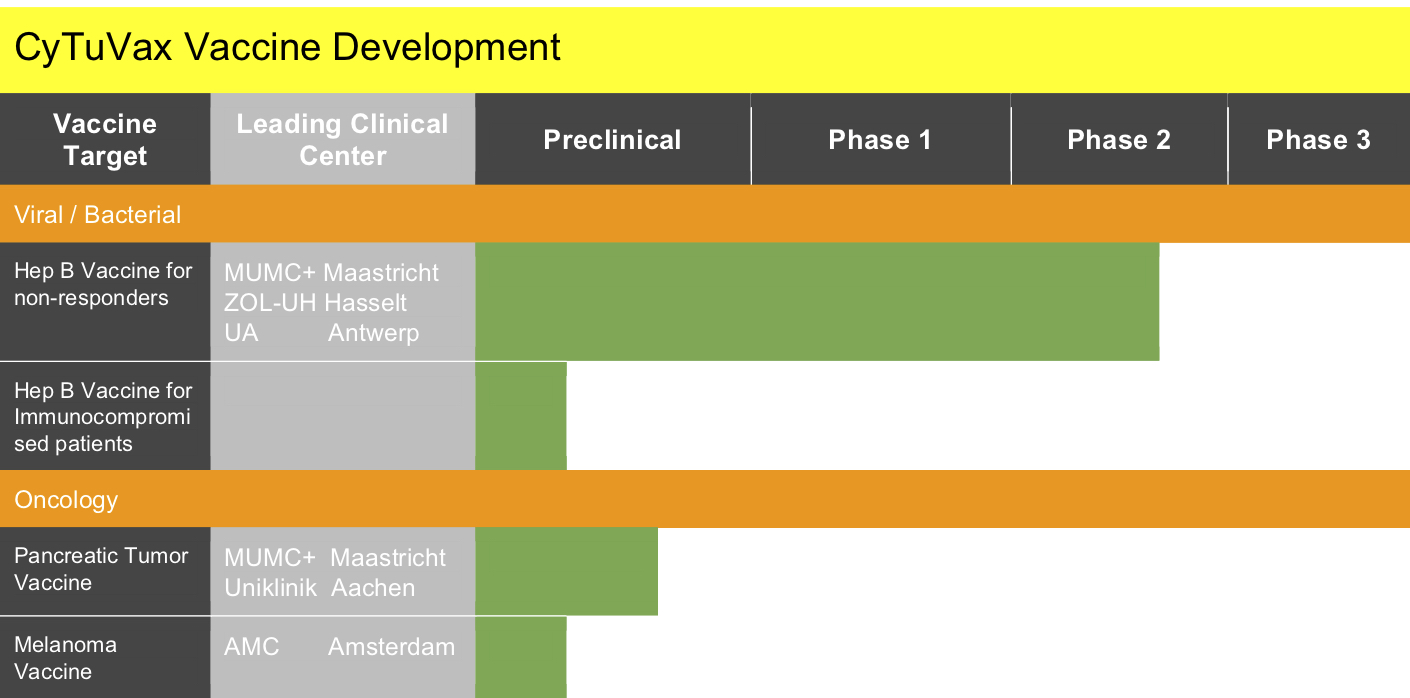
Phase 1 & 2 Clinical Trials on Safety and Immunogenicity of HBAI20 Hepatitis B Vaccine
Worldwide, people are suffering from the consequences of Hepatitis B (HB) virus infection, such as liver cirrhosis and liver cell carcinoma. Currently available vaccines are protective in most of the vaccinated population. However, a small part of the population does not respond to these vaccines and they are called (non-responders). The innovative adjuvant AI20 has been developed by CyTuVax to improve the standardized Hepatitis B vaccine for the protection of non-responders.
Clinical Trial phase 1 ClinicalTrials.gov #NCT02540538
Clinical Trial phase 2 ClinicalTrials.gov #NCT03415672