Vaccine Adjuvant
CyTuVax has developed a vaccination technology that applies depot-attached cytokines as adjuvant. This adjuvant is capable of eliciting powerful immune responses against viral and cancer targets, and can be applied in both prophylactic and therapeutic vaccines.
The personalized tumor vaccines under development consist of depot- attached aggregated cytokine molecules as adjuvant. The personalized vaccine target consists of mRNA and/or tumor cells and/or overlapping long peptides (OLPs).
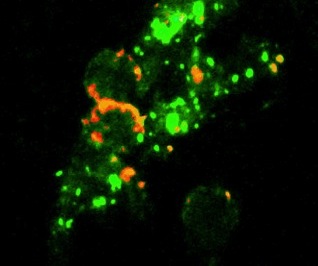
The CyTuVax vaccine: cytokine aggregates (green)
tumor cells (red) and tumor cell fragments (red).
The first distinctive feature of the vaccine adjuvant is the highly improved antigen presentation by dendritic cells (DCs). After injection of the vaccine, an inflammatory process is created at the injection site, that attracts large numbers of DC precursors. These cells mature in-situ, thereby processing the mRNA/ tumor cells/ OLPs and presenting these as tumor peptides on their cell membrane.
The second distinctive feature is that the matured DCs are capable of taking up cytokine aggregates with their low affinity receptors and carry these to the nearest lymph node. Within the lymph node the DCs are capable of forcefully activating , immune cells under the influence of the cytokine aggregates to induce powerful immune responses.
These are unique features of the CyTuVax vaccine technology, as no other vaccination technology is capable of carrying and depositing cytokine- aggregates directly into the lymph node to stimulate the activation of innate immune cells.